How Long Does The Virus Live In Bird/ Animal Droppings
Avian polyomavirus (APV) causes disease in young parrots. In that location are two forms of the affliction based on affected species: budgerigar fledgling affliction and non-budgerigar polyoma infection. Both are characterized past peracute to astute death of preweaned neonates. Clinical signs occur 7–x days after exposure and include lethargy, ingather stasis, and death within 24–48 hours. Surviving budgerigars >iii weeks one-time often exhibit plume dystrophy (French molt or plumage dusters). Older nonbudgerigar psittacines may have subclinical disease or hemorrhages and coagulopathies. Developed birds typically are resistant to infection; they will seroconvert and shed the virus for up to ninety days, so clear the infection. Antemortem diagnosis is accomplished with DNA probes of cloacal swab and blood samples. Asylum command methods include not housing budgerigars or lovebirds with other species, strict hygiene, limiting traffic in the nursery, and strict quarantine and testing of new birds. A vaccination is bachelor.
The typical presentation of budgerigar fledging disease is a well-fleshed juvenile, but before fledgling age, with acute onset of lethargy, crop stasis, and decease within 24–48 hours. Other clinical signs are cutaneous hemorrhage, abdominal distention, and plume abnormalities. Surviving budgerigars >3 weeks old often exhibit feather dystrophy (French molt or feather dusters). In other species of psittacines <4 months quondam, the infection is often fatal. Older nonbudgerigar psittacines may have subclinical illness or hemorrhages and coagulopathies.
Antemortem diagnosis is accomplished with Dna probes of cloacal swab and blood samples and virus-neutralizing antibody tests of blood samples to identify birds with previous viral exposure. Diagnosis in a flock setting is typically based on clinical signs, signalment, and necropsy findings.
Gross necropsy findings in deceased chicks often include pale skeletal musculature and subcutaneous ecchymotic hemorrhages. The kidneys and liver are enlarged and may be pale, congested, and mottled, or accept pinpoint, white foci. Petechial or ecchymotic hemorrhages may as well exist present on viscera, particularly the heart. The heart is sometimes enlarged and may show hydropericardium. Intranuclear inclusion bodies are often seen in the liver, kidneys, middle, spleen, bone marrow, uropygial gland, skin, feather follicles, etc.
Aviary control methods include avoiding the housing of budgerigars or lovebirds on bounds where other species are bred, adhering to standard hygiene procedures, preventing access to the nursery by visitors, and not introducing birds into the aviary without 90 days quarantine and testing. Eliminating APV infection from an infected budgerigar aviary is challenging. Showtime, all breeding must exist stopped for 6 months. The presence of infected neonates, fledglings, and adults propagates the disease. During this time, developed birds are moved to a noninfected area while the entire aviary is disinfected. Nest boxes should be disinfected or discarded and replaced. After 6 months, adult breeding birds can be returned to a clean aviary and breeding resumed.
Pet shop prevention should include separating neonates from different sources, purchasing birds from sources where polyomavirus testing and vaccination are performed, and ideally, not purchasing or selling unweaned birds.
Treatment is supportive care. A vaccine is available. For breeding birds, ii doses of the vaccine are administered at a two-calendar week interval; this should exist done off-season. The manufacturer recommends administration of the first dose when the chick is >35 days old, with a booster vaccination in 2–3 weeks.
Psittacine bill and feather illness (PBFD) is caused by a psittacine circovirus. The virus was first recognized in the 1970s in cockatoos with pecker and feather lesions. Since and then, it has been recognized in most species of parrots and likewise in Passeriformes and Columbiformes. It primarily affects young parrots. Infected birds present with abnormal feathers, beak lesions, and immunosuppression. Infected birds shed virus in their feathers, plume dander, feces, and oral secretions. Transmission occurs by inhalation and/or ingestion of the virus and tin can occur vertically. The virus is very stable in the environment, then fomites are a notable source of infection. Diagnosis is based on clinical signs and results of PCR testing. Treatment is supportive care. Due to its contagious nature and final outcome, humane euthanasia may be warranted. Command involves strict hygiene, testing, and quarantine of all new birds.
The name is non as representative of the current typical clinical presentation, which often does not include beak abnormalities and is less likely to have the severe, classic plume abnormalities seen in cockatoos when the disease was kickoff documented. Apply of screening PCR assays has greatly decreased the prevalence of the virus in captive bred Cacatua spp. However, disease is still seen in African grey parrots, Eclectus parrots, lovebirds (Agapornis), lorikeets, and other species, but is rare. The natural infection appears to occur primarily in juvenile birds, with few instances of clinical infection seen in birds >3 years erstwhile.
In the classic PBFD infection, the get-go indication of the presence of disease is a lack of powder downwardly on the bill. The virus causes abnormal germination of growing feathers and immunosuppression. Feathers are pinched or clubbed at their base of operations and may have hemorrhage within the developing shaft. The feathers autumn out easily and grow dorsum slowly or not at all. The distribution of afflicted feathers depends on the age of the bird and the phase of the molt when infected. Paint loss may occur in colored feathers. The bird may live with these lesions for months to years.
As the affliction progresses, the immune organisation is affected, and almost birds die of secondary infections. A peracute grade of the affliction occurs in immature birds, which develop enteritis and pneumonia, lose weight, and die. African greyness parrots may develop a pancytopenia, considering the virus attacks the bone marrow. These birds dice suddenly with viral inclusions in the thymus, bursa, and bone marrow.
Diagnosis of psittacine beak and feather illness is based on clinical appearance; results of PCR assay of feces, feather dander, or blood; and biopsy of affected plume follicles showing basophilic intracytoplasmic inclusions. Testing by PCR assay may detect infection in birds that yet announced healthy. These birds may subsequently become sick or may mount an constructive response to the virus. Because of the stability of the virus, PCR analysis tin can also be used for ecology testing. Quarantine and retesting are recommended for PCR-positive, asymptomatic birds. At necropsy, affected birds frequently accept no gross lesions internally, simply intranuclear or intracytoplasmic inclusions may exist seen histologically in the feathers, bursa, thymus, liver, or other organs.
There is no specific treatment for PBFD, and handling of infected birds is supportive. The contagious nature of PBFD and its mostly final outcome in clinically affected birds warrant isolation and eventual euthanasia in most clinical cases. Strict hygiene with attending to dust command, screening protocols including PCR testing of both birds and the environment, and lengthy quarantines are highly recommended in breeding facilities with susceptible species. All new susceptible birds should be tested before introduction to the aviary. In infected breeding colonies, removing all eggs for cleaning and bogus incubation may besides be required. Since the development of a PCR assay, prevalence of the disease has decreased.
Psittacine herpesvirus is an alpha herpesvirus that is the causative amanuensis of Pacheco's affliction and internal papillomatosis in parrots. Pacheco's illness causes a viral hepatitis seen predominantly in New Earth species (Amazon parrots, macaws, and conures).
Internal papillomatosis occurs in parrots that have survived Pacheco's affliction. Papillomatosis is virtually commonly observed in macaws, Amazon parrots, conures, and Hawk-headed parrots. Affliction is associated with stress, which can crusade clinically good for you carriers to shed virus and initiate infection in susceptible birds, equally often occurs during introduction of new birds, relocation, or in those with underlying illness or during breeding. It is spread by directly contact, droplets, or fecal contagion of food or water, with an incubation period of 3–14 days. The effect of the infection depends on the genotype of the virus, the species of bird infected, and the bird'due south overall health. Infected birds get chronic carriers and will remain persistently infected and intermittently shed the virus throughout their lives. Old World species are less likely to be either inapparent carriers or clinically susceptible. Patagonian species and some Aratinga spp may be natural hosts in the wild, and some individuals of these species may asymptomatically shed virus when stressed. Other species can too human activity as carriers.
Concluding signs of Pacheco's illness include acute death in well-fleshed birds and bright yellowish urates with scant feces. Other clinical signs are diarrhea, green urates, sluggishness, regurgitation, weakness, and depression.
Diagnosis of Pacheco's disease in a live bird can be done past Dna probes of combined oral and cloacal swabs and blood samples. Increases in plasma AST activity and marked leukopenia have been reported.
Because of the acute nature of the affliction, histologic lesions may non exist evident. However, most affected birds will have hepatomegaly, splenomegaly, and renomegaly. The liver may be mottled or grossly discolored. Ecchymotic and petechial hemorrhages may be nowadays on the pericardium and within the mesenteric fat. Intranuclear inclusions are seen histologically in the liver, spleen, intestinal epithelium, and pancreas.
In addition to supportive intendance, acyclovir (80 mg/kg, three times a day, or 400 mg/kg in feed) can exist used during an outbreak; nevertheless, the risk of increased transmission because of handling is great. Autogenous vaccines have been developed during outbreaks and have effectively decreased morbidity and mortality. An inactivated vaccine is available.
The lesions of papillomatosis are predominantly present in the oral and cloacal mucosa but may also be institute internally in the intestinal tract, or less commonly, in the conjunctiva or bursa. Owners usually first notice blood from a papilloma in the droppings, and/or the papilloma prolapses through the cloaca. Lesions may exist mild or severe (ulcerated and haemorrhage) and ofttimes wax and wane. Ulcerated lesions may need to be cauterized or surgically removed, although they typically recur. Treatment is supportive, such as analgesics, cautery, and antimicrobials to foreclose secondary infection. Antiherpesviral drugs are not curative and exercise not announced to impact the course of disease.
Avian bornavirus (ABV) is a neurotropic virus that causes proventricular dilatation affliction (PDD). PDD is a progressive neurologic disease that uniquely affects the nervous organisation and is fatal in one case clinical signs develop. PDD, too known equally macaw wasting disease, neuropathic ganglioneuritis, lymphoplasmacytic ganglioneuritis, psittacine encephalomyelitis, and avian ganglioneuritis, was starting time recognized in the late 1970s in macaws imported into the US and Germany. The illness primarily affects macaws, conures, and African grey parrots, although all parrots are considered susceptible. Avian bornavirus has been reclassified. The two species known to crusade PDD in parrots are psittaciform bornavirus 1 and 2 and include parrot bornavirus (PaBV) genotypes i–eight. PaBV-2 and PaBV-4 are the most commonly reported genotypes in parrots.
The most mutual presentation of afflicted birds is chronic weight loss (often following an initial increase in appetite), passage of undigested food (most hands recognized when whole seeds are institute in the debris), and regurgitation. A dilated proventriculus may be seen radiographically. GI tract signs often reflect pathology of the last ganglia of the vagus nerve (cranial nervus X). Secondary bacterial or fungal infections of the nonmotile proventriculus tin can result in chronic infections and sepsis. Myocarditis related to avian bornavirus/PDD has been reported. Neurologic signs (convulsions, tremors, weakness, clutter, blindness) may occur in some species, with or without concurrent GI signs. Avian bornavirus is shed in the urine also as the carrion, and some birds may experience polyuria. Clinical signs may be slowly progressive or develop acutely. Outbreaks are sporadic, with a low morbidity and a loftier mortality.
Avian bornavirus/PDD is contagious, but the bodily mode of transmission is not articulate. The spread of PaBV and PDD has been well documented, detailing how diseases spread subsequently introduction of an developed bird with fatal PDD into an aviary. Because ABV tin can be detected in debris (feces and urine), the fecal-oral route of transmission is considered the near likely, although a recent article investigating different infection routes of parrot bornavirus in cockatiels revealed that transmission of PaBV by oral or intranasal routes was unsuccessful. A second article indicated that transmission of PaBV by direct contact is inefficient in immunocompetent, fully fledged birds.
PaBV is widely distributed in convict and wild bird populations, and the infection is common amidst captive psittacines in Northward America and Europe. Still, infection does not always cause PDD. Many salubrious or subclinical carriers of several species take been documented. In studies in Europe and in North America, every bit many as fifteen%–xl% of salubrious parrots are positive for PaBV. Vertical transmission has non been proved but seems likely.
A presumptive diagnosis can be made based on history, clinical signs, and radiographic evaluation. Survey films, radiographs of the GI tract with contrast, and fluoroscopy can aid in diagnosis. The proventriculus is often severely dilated. Dissimilarity studies often reveal delayed transit times throughout the GI tract. Decreased motility may pb to gram-negative or clostridial enteritis. Serum chemistry and hematology are typically normal, although at that place may be an increased CPK concentration, mild anemia, heterophilia, and/or hypoproteinemia.
PCR assay of the cloaca or feces is the virtually common test performed. Because shedding of the virus is intermittent, one negative result on a fecal or cloacal PCR test does not exclude disease. Testing at least three times at monthly intervals, with all three tests being negative, is the best evidence to declare a bird negative for ABV. Serologic assays such as ELISA, IFA, and Western blot can also confirm exposure but may non find early infections, because some PaBV-infected birds do not develop a detectable antibody response until late in the illness process. The assessment of PaBV infection and the diagnosis of PDD is difficult because, although PaBV infection is common, development of clinical PDD is rare. Thus, tests that simply notice the presence of PaBV may not be particularly useful for the antemortem diagnosis of PDD.
Some researchers take proposed serologic testing for antiganglioside antibodies, stating that information technology appears to more accurately detect clinically affected birds. Antiganglioside antibodies are used as markers of immune-mediated affliction and are triggered by a variety of pathogens. Other studies accept not supported this.
Definitive diagnosis is confirmed by histopathology with characteristic myenteric ganglioneuritis lesions in the ingather, ventriculus, or adrenal gland. Ventricular and adrenal gland biopsies are risky and seldom performed. Crop biopsies are reported to exist diagnostic of PDD in simply about 30%–35% of cases. Failure to detect lesions in the ingather does non exclude affliction. Proventricular biopsies in affected birds are non done routinely considering the proventriculus is prone to dehiscence. Often, PDD is diagnosed at necropsy. Histologic examination should exist done on the ingather, proventriculus, ventriculus duodenum, adrenal gland, heart, kidney, spleen, and brain.
Interpretation of diagnostic tests for PDD:
-
A choanal and cloacal RT-PCR with a serologic assay, recognizing that ane negative test on all does not exclude infection
-
A bird that tests positive on both PCR assay and serology should be considered positive and housed separately from birds with negative results
-
A bird positive by PCR only should exist retested in 4–6 weeks and housed separately from other birds until it has 3 negative tests or is confirmed positive on additional tests
-
A bird positive on a serology test but should be considered positive and housed separately from birds testing negative
-
Positive ABV PCR and serology results in a healthy bird practice not predict future clinical illness with PDD
Treatment for PDD includes providing supportive care, including easily digestible foods, and may exist aided by administration of an NSAID Nonsteroidal Anti-inflammatory Drugs in Animals The importance of pain management and the utilise of nonsteroidal anti-inflammatory drugs (NSAIDs) in animals has increased dramatically in contempo decades, and use of NSAIDs in companion animals... read more  (eg, meloxicam, celecoxib, or robenacoxib). Supportive care may include GI prokinetic agents such as cisapride and metoclopramide to improve GI tract transit. Antimicrobials or antifungals may be necessary for secondary infections. Flaxseed oil at 0.ane mL/kg, PO, daily or a veterinary omega 3 and vi fatty acrid supplement at 0.22–0.44 mL/kg, PO, daily have been advocated and used to reduce inflammation.
(eg, meloxicam, celecoxib, or robenacoxib). Supportive care may include GI prokinetic agents such as cisapride and metoclopramide to improve GI tract transit. Antimicrobials or antifungals may be necessary for secondary infections. Flaxseed oil at 0.ane mL/kg, PO, daily or a veterinary omega 3 and vi fatty acrid supplement at 0.22–0.44 mL/kg, PO, daily have been advocated and used to reduce inflammation.
Anecdotally, stress has been a gene in development of clinical signs; therefore, methods to reduce stress should be implemented, including practicing expert hygiene, providing nutritionally adequate diets, and avoiding overcrowded weather. Reproductive stress may also exacerbate clinical signs. Leuprolide or the deslorelin implant have been used to reduce reproductive hormones. Gabapentin has been recommended for central or peripheral nervous system signs such as seizures or ataxia. The dose for gabapentin is 10–25 mg/kg, PO, every 12 hours. For birds that self-mutilate, the gabapentin dose is 50 mg/kg, PO, every 12 hours. To date, no antivirals have been constructive in treatment or prevention of ABV.
NSAIDs for PDD treatment:
-
Meloxicam 0.five–1 mg/kg, PO or IM, every 12 hours
-
Robenacoxib 7–x mg/kg, IM, every 7 days × iv weeks then every four weeks
-
Celecoxib 15–30 mg/kg, PO, every 12 hours
Isolation of positive birds is important in disease prevention. Testing by PCR and serology (a minimum of three tests washed iv–vi weeks apart) and separating birds that examination positive from those that exam negative is a recommended control measure. However, the number of fake-negative tests (because of intermittent shedding) makes this a long and potentially difficult task, and clinically healthy birds that test positive for ABV should not be euthanized. ABV is not a long-lived virus in the environment; therefore, practiced hygiene and ultraviolet light tin can assistance to limit spread of disease in a home or aviary setting.
The command of ABV in aviaries and homes requires a multimodal approach:
-
Practicing expert hygiene
-
Housing birds outdoors when temperatures allow
-
Avoiding overcrowding and stress and providing expert nutrition
-
Isolating of all new, ill, or ABV-positive birds
-
Testing existing and newly presenting birds using both serology and multiple (up to three) PCR assays, and housing birds separately based on results.
-
With the possibility of vertical transmission, chicks from infected birds should be incubator hatched and hand raised, separated from other noninfected birds and monitored for evolution of illness.
-
Due to the intermittent shedding and inconclusive serology results, it may take years of testing and separating birds to obtain ABV-negative aviaries.
-
There is no vaccine available. Vaccine studies are in their infancy.
The three clinical forms of poxvirus infection are:
-
Cutaneous form or "dry pox" –wart-like lesions typically on the face, pecker, and legs (most common course)
-
Diphtheritic form or "wet pox" –lesions on the mucosa of the oral cavity and respiratory tract
-
Septicemic "generalized pox" –internal lesions affecting the respiratory and GI tracts, causing systemic disease
Poxviruses are large DNA viruses that induce intracytoplasmic, lipophilic inclusion bodies (Bollinger bodies) in the epithelial cells of the integument, respiratory tract, and oral crenel. All birds are considered susceptible to poxvirus infection, simply many companion and aviary birds are rarely exposed to a susceptible strain. In pet bird practice, veterinarians will generally run across only canary and , pigeon poxviruses, and fowlpox Fowlpox , which have specific host ranges. Poxviruses are environmentally stable, increasing the likelihood that a viable organism will come into contact with a susceptible host. Poxviruses cannot penetrate intact skin, and a break in the skin or mucous membrane must be present for infection to occur.
Poxvirus infection may crusade cutaneous, diphtheritic, or systemic infections based on the strain of virus, route of exposure, afflicted species, and historic period and wellness of the bird. The cutaneous course appears as nodular proliferations or wartlike lesions on the unfeathered skin effectually the eyes, pecker, nares, and legs. The diphtheritic class is characterized by lesions on the mucosa, natural language, pharynx, and larynx. The septicemic form is characterized by a ruffled appearance, depression, cyanosis, anorexia, and wartlike tumors of the skin. The cutaneous grade is most normally seen in psittacines and raptors.
Clinical signs depend on the form of illness, location of the lesions (eye, oral, ear), and overall health of the bird and may include lethargy, respiratory distress, partial blindness, difficulty eating, weight loss, emaciation, and peel lesions.
Diagnosis of poxvirus infection is typically confirmed through history, physical examination findings, and histologic findings of Bollinger bodies in affected tissues.
Treatment is usually nonspecific and may include supportive care, fluids, parenteral vitamin A, ophthalmic ointments for heart infections, assisted feedings, and antimicrobials to prevent or treat secondary infections. Lesions on the peel may need daily cleaning.
Manual is via insect vectors (mosquito bites) or other entry through breaks in the pare. Therefore, mosquito control and indoor housing are vital to prevent outbreaks. Vaccines for canarypox, fowlpox, and pigeonpox are available but are specific for their host species.
Viscerotropic velogenic Newcastle disease Newcastle Disease and Other Paramyxovirus Infections (VVND), caused by a paramyxovirus grouping 1, affects near avian species and is an important threat to the poultry industry. It is rare in captive-bred parrots housed indoors. Transmission is by respiratory aerosols, fecal contagion of food or water, direct contact with infected birds, and fomites.
Birds may exist asymptomatic or dice acutely. Clinical signs include low, anorexia, weight loss, sneezing, nasal belch, dyspnea, conjunctivitis, bright yellow-green diarrhea, ataxia, head bobbing, and opisthotonos. In prolonged cases, unilateral or bilateral wing and leg paralysis, chorea, torticollis, and dilated pupils besides may be seen. Primary differential diagnoses include other paramyxoviruses Other Paramyxovirus Infections Avian polyomavirus (APV) causes affliction in immature parrots. There are two forms of the disease based on afflicted species: budgerigar fledgling disease and non-budgerigar polyoma infection. Both... read more than 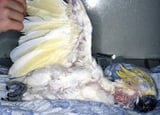 (non-Newcastle), psittacine proventricular dilatation syndrome Avian Bornavirus / Proventricular Dilatation Affliction Avian polyomavirus (APV) causes disease in immature parrots. In that location are two forms of the disease based on afflicted species: budgerigar fledgling disease and non-budgerigar polyoma infection. Both... read more
(non-Newcastle), psittacine proventricular dilatation syndrome Avian Bornavirus / Proventricular Dilatation Affliction Avian polyomavirus (APV) causes disease in immature parrots. In that location are two forms of the disease based on afflicted species: budgerigar fledgling disease and non-budgerigar polyoma infection. Both... read more  , and heavy metallic toxicosis Heavy Metal Toxicosis Pb toxicosis is ordinarily reported in companion birds also as in free-ranging avian species, peculiarly waterfowl. In pet birds, toxicosis frequently occurs from ingestion of metal in the home... read more . Lesions include hepatomegaly, splenomegaly, petechial or ecchymotic hemorrhages on serosal surfaces of all viscera and air sacs, airsacculitis, and excess straw-colored peritoneal fluid. Diagnosis is traditionally via viral isolation, but agar gel immunodiffusion tests that can exist performed on whole blood or serum are bachelor.
, and heavy metallic toxicosis Heavy Metal Toxicosis Pb toxicosis is ordinarily reported in companion birds also as in free-ranging avian species, peculiarly waterfowl. In pet birds, toxicosis frequently occurs from ingestion of metal in the home... read more . Lesions include hepatomegaly, splenomegaly, petechial or ecchymotic hemorrhages on serosal surfaces of all viscera and air sacs, airsacculitis, and excess straw-colored peritoneal fluid. Diagnosis is traditionally via viral isolation, but agar gel immunodiffusion tests that can exist performed on whole blood or serum are bachelor.
Only symptomatic treatment is possible and thus not advised. If suspected, VVND must be reported to advisable federal and state authorities.
In that location are several less pathogenic strains of paramyxovirus. Paramyxovirus groups ii and 3 are endemic in aviculture. Paramyxovirus group ii causes mild disease in passerines and a more serious illness in psittacines. Clinical signs in psittacines include tracheitis, pneumonia, and enteritis. Paramyxovirus group 3 is reported almost frequently in Neophema spp, lovebirds, and gouldian finches and typically causes balmy affliction. Clinical signs may be absent-minded, and disease results in acute death. In disease of longer duration, respiratory signs, pancreatitis, and torticollis may occur.
Diagnosis is the aforementioned as for paramyxovirus group 1. Treatment for paramyxovirus groups two and 3 infections is supportive intendance. The vaccine for paramyxovirus group i should non be used in psittacines, considering it can cause fatal reactions.
W Nile virus (WNV) infection is an arthropod-borne virus in the genus Flavivirus (family Flaviviridae). WNV was first reported in birds in the US in August 1999. WNV has been reported in >320 species of birds. The American crow (Corvus brachyrunchus) and other corvids have suffered especially loftier morbidity and mortality. Other afflicted species include canaries, psittacines, and raptors. Although psittacines appear to be somewhat resistant, the disease has been reported in parakeets, cockatoos, conures, rosellas, caiques, lorikeets, and a King parrot. Affected parrots take been adults housed outdoors with documentation of musquito populations present. Mosquitoes (Culex spp) are the principal vectors of disease.
Clinical signs include depression, anorexia, weight loss, head tremors, ataxia, blindness, seizures, and death. Juvenile birds are the nigh commonly afflicted. Ophthalmologic findings in raptors are anterior uveitis, exudative chorioretinal lesions, and chorioretinal scarring.
Antemortem diagnosis can be hard. Initial diagnosis may be based on clinical signs, species, and age; withal, many diseases may cause like clinical signs. Serologic tests (serum neutralization) may signal antibody response to infection. Paired samples submitted 2 weeks apart may reveal a rise in antibody levels and requite a more definitive diagnosis. Developed birds may accept loftier circulating antibody levels in endemic areas. PCR assay is available. Diagnosis is frequently determined at necropsy. The brain and kidney are the preferred tissues to submit for histopathologic examination.
There is no specific treatment for WNV in birds. Some birds may better with supportive care (fluids, feeding, antimicrobials/antifungals equally needed) and time. A vaccination protocol using a recombinant vaccine has been successful in some birds. The recommendation is vaccination of captive birds 2–iv weeks earlier mosquito season, with a booster 3 weeks after the initial dose.
For control, during the mosquito season, birds should be housed indoors or in completely covered outdoor facilities. Mosquito netting and mosquito traps should be used, and any standing or stagnant water sources eliminated.
Avian influenza Avian Flu is caused by an orthomyxovirus. Because of the zoonotic potential of some strains and the contempo discovery of new mutations, this virus may become a more important pathogen. Both the zoonotic potential and the economic effects on the poultry industry are causes for concern.
Source: https://www.msdvetmanual.com/exotic-and-laboratory-animals/pet-birds/viral-diseases-of-pet-birds
Posted by: danielalmom1995.blogspot.com

0 Response to "How Long Does The Virus Live In Bird/ Animal Droppings"
Post a Comment